Abstract
Background : Continuous infusion of factor VIII (FVIII) is a more cost-effective method for treating hemophilia A than intermittent bolus injection, especially when undergoing surgery. The activity of the factor 8 concentrates may gradually decreases over 24 hours at room temperature, making it difficult to maintain a desired appropriate blood concentration. However, there are no specific guidelines for precise method and duration of continuous intravenous. administration of factor VIII products. In this study, we evaluate in vitro factor VIII activity during 24 hours after reconstitution with diluent fluid in various conditions to propose an effective continuous i.v. infusion method.
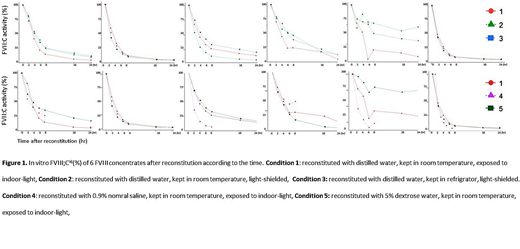
Method : A total 6 of coagulation factor VIII concentrates were used (2 plasma derived FVIII products and 4 recombinant FVIII products) - Greengene-F®,Advate®, Xyntha®, monoclateP®, Koginate®,Immunate®. Each drug was dissolved in the enclosed water for injection according to the manufacturer's instruction. After 30 minutes of stabilizing, each drug was further diluted in sterilized distilled water or normal saline or dextrose water was adjusted to the same concentration for this experiment. Then, the drugs were stored at refrigeration or room temperature with exposure to light or light-shielded. In vitro FVIII:C was measured at five time points (0, 2, 4, 6, 8, and 24 hours after reconstitution) with a one-stage clotting assay a CS5100 coagulation analyzer system from Sysmex. Then, the factor VIII activity of each time was statistically compared with activity of 0 hour.
Results: With all experimental conditions, FVIII activities of all 6 drugs persistently decreased over the course of 24 hours (p <0.001). There was a significant difference in the degree of decrease in activity over time depending on the type of drug (p <0.001), the activity after 2 hours is 47.58 to 90.76 %, the activity after 4 hours is 31.58 to 86.21 %, the activity after 8 hours is 6.49 to 77.03 %, the activity after 18 hours is 2.24 to 75.49 %, the activity after 24 hours is 1.23 to 73.63%. Two hours after the dilution, the FVIII activity decreased to 80% or less with 3 of 6 drugs. After 4 hours, 5 of 6 drugs had the FVIII activity of 80% or less, and after 6 hours the FVIII activity decreased to less than 80% in all of 6 drugs. There was a difference in the degree of decrease in activity depending on the presence or absence of shading and refrigeration, and diluent solutions (normal saline or dextrose water instead of distilled water), but there was a difference between the drugs and consistent correlation was not observed.
Conclusions : When the FVIII concentrates were diluted, the activity of each of the six drugs decreased significantly over the 24 hours in vitro, and the degree of decrease was significantly different according to the exposed conditions although there was a difference by drug. Based on the results of the continuous decrease in FVIII activity over time, it is not appropriate to dissolve a 24-hour dose in a large amount of diluted fluid at one time and continuously infuse during 24 hours. Replacing the concentrate before 2 to 6 hours after dilution is probably a better method to maintain desired FVIII activity in patients with hemophilia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal